Pipeline
Building a pipeline of advanced dermatological drugs
With a focus on expediting the development of effective dermatological solutions, we leverage our expertise to identify, evaluate, develop, and commercialize products that have the potential to address a variety of both rare and common skin conditions.
Rigorous testing and methodical analysis drive us forward, ensuring that only the most effective and safest treatments progress through the pipeline. Through this comprehensive approach, we strive to make these assets successful not just in the lab, but also where it matters most—in the lives of patients.
SGT-610
Patidegib gel, 2%
Gorlin syndrome
SGT-210
Topical erlotinib
Pachyonychia congenita and other rare skin conditions
Completed Phase I
TWYNEO®
Tretinoin and benzoyl peroxide cream, 0.1%/3%
Acne vulgaris
Galderma partnership in the US
Benzoyl peroxide cream, 5%
Inflammatory lesions of rosacea
Galderma partnership in the US
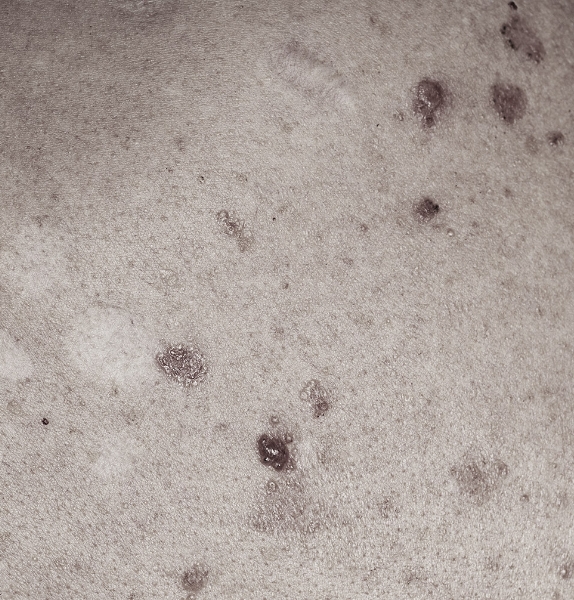

SGT-610
Our lead candidate, SGT-610, is a topically applied 2% patidegib gel that is currently being developed for the prevention of new basal cell carcinoma (BCC) lesions in patients with Gorlin syndrome.
Harnessing deep experience in clinical trial design for topical dermatologic drugs, we aim to advance SGT-610 toward FDA approval, ultimately offering patients with Gorlin syndrome the first-ever therapy that could prevent new BCC lesions.
BCC lesions and Gorlin syndrome
BCC growth is driven by abnormal activation of hedgehog signaling, a highly conserved evolutionary cellular signaling pathway that has significant implications for the development of skin and other types of cancers.1 Dysregulated hedgehog signaling is a pivotal molecular abnormality underlying the development of BCC lesions.2
Hedgehog inhibitors (HHIs) are a promising class of cancer-fighting therapeutics with the potential to prevent the development of new BCC lesions—the most common manifestation of Gorlin syndrome.3
Recognizing the potential of HHIs to be a preventive treatment for BCC lesions in patients with Gorlin syndrome, we acquired patidegib—a biologically validated HHI. SGT-610 is the first-ever topically applied patidegib gel, leveraging a unique formulation that carries minimal risk for the systemic adverse events typically associated with oral HHIs.

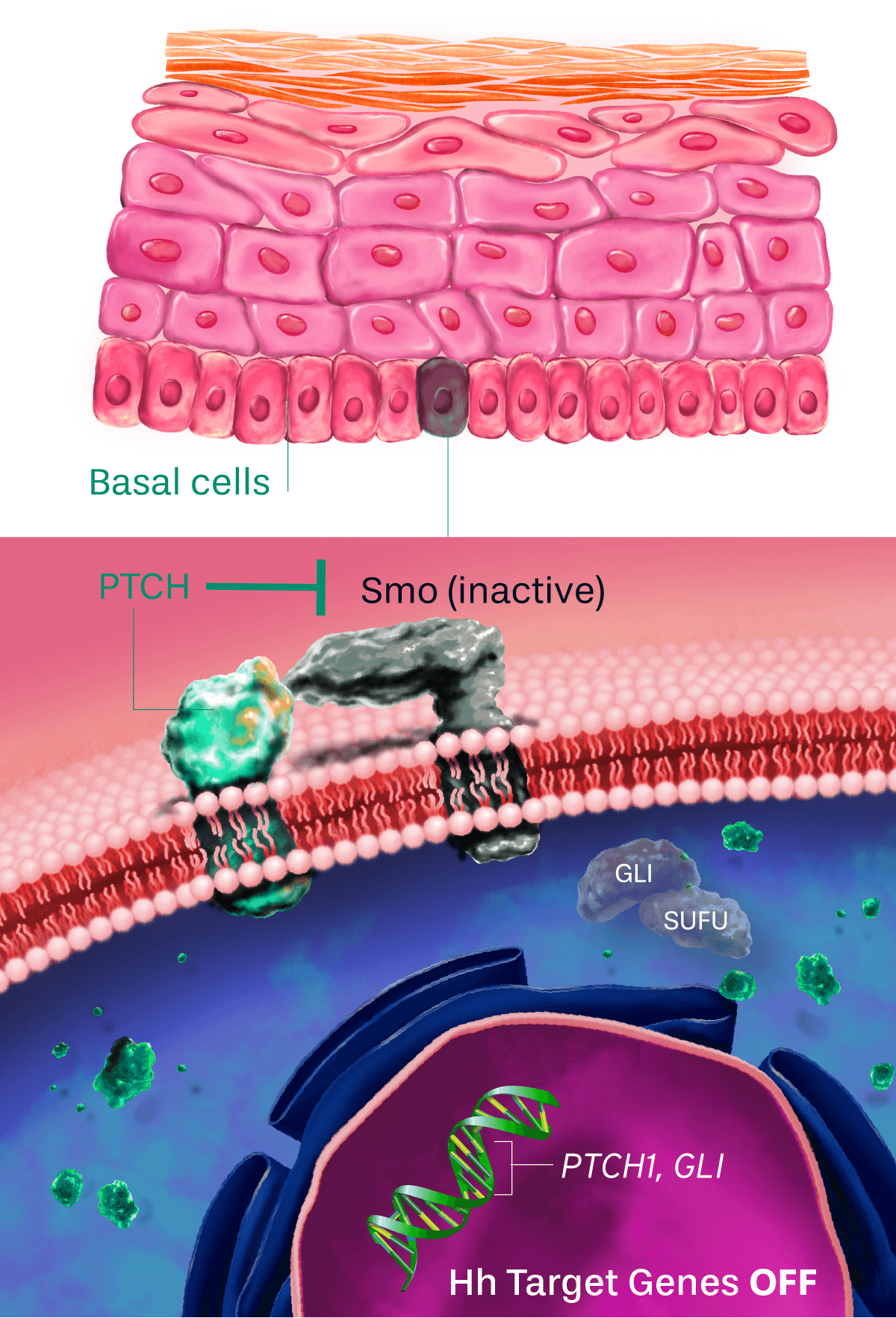
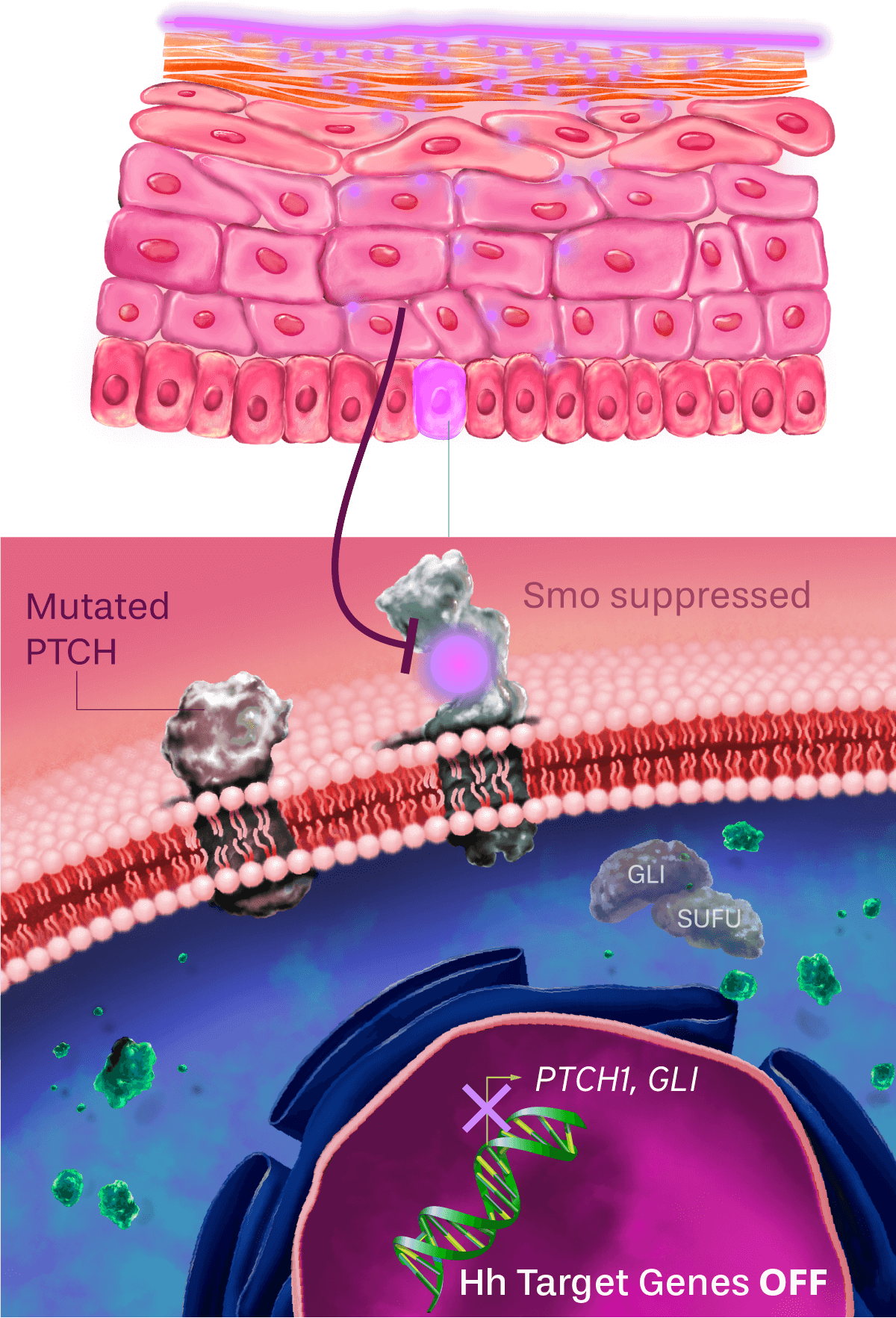
Patidegib Mechanism of action
Normal pathway
IN THE ABSENCE OF HEDGEHOG SIGNALING, THE PATHWAY IS OFF
The hedgehog signaling pathway plays an important role in cellular function and tissue development. PTCH inhibits the activity of Smo, causing pathway inhibition. The inhibition of Smo is lifted when the hedgehog ligand binds to PTCH, continuing to promote downstream signaling.

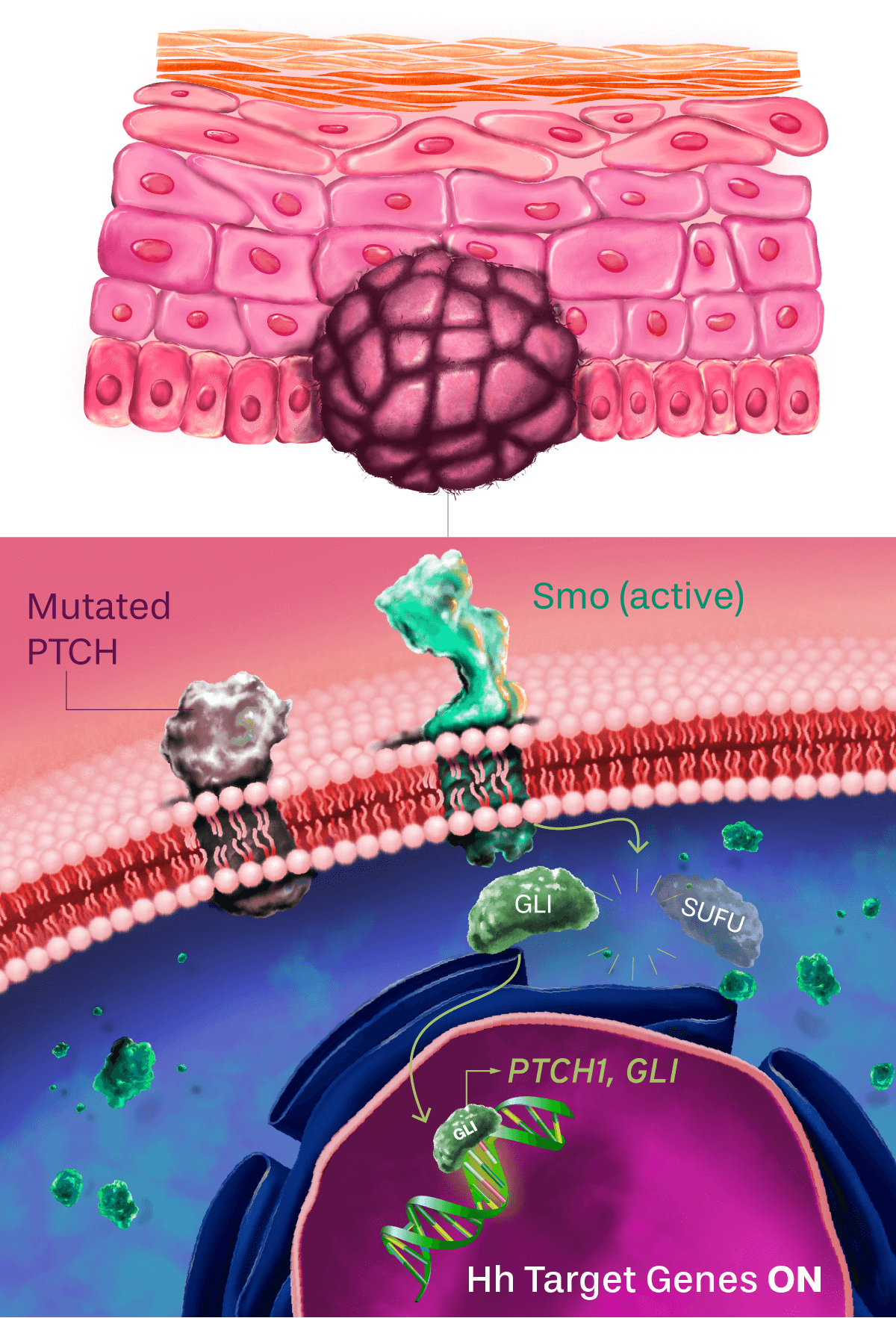
Dysregulated pathway in basal cell carcinoma
HEDGEHOG SIGNALING PATHWAY Constantly on
The PTCH inactivating mutation results in constitutive activation of the hedgehog signaling pathway in all basal cell carcinomas (BCCs) in patients with Gorlin syndrome. As a result, BCC cells divide uncontrollably.

Topical patidegib
HEDGEHOG SIGNALING PATHWAY OFF
Patidegib is designed to replace PTCH activity by inhibiting Smo and blocking the hedgehog signaling pathway, so the cells stop dividing. Inhibiting the hedgehog signaling pathway prevents new BCCs from growing.



Optimizing clinical development for improved outcomes
Prior clinical testing results for SGT-610 presented the opportunity for improved trial design enhancements. We consulted with key opinion leaders in dermatology to design a rigorous phase 3 trial to study the safety and efficacy of SGT-610, improving on previous approaches by focusing exclusively on patients with relevant mutations and a high burden of BCC lesions.
The phase 3 trial started in Dec 2023.


Accelerating the path of accessibility to patients
Patidegib was granted Orphan Drug designation by the United States Food and Drug Administration (FDA) and the European Commission, as well as Breakthrough Therapy designation by the FDA. These designations exemplify the remarkable therapeutic potential of patidegib and propel us closer to fulfilling our mission to deliver a noninvasive treatment to patients with Gorlin syndrome.
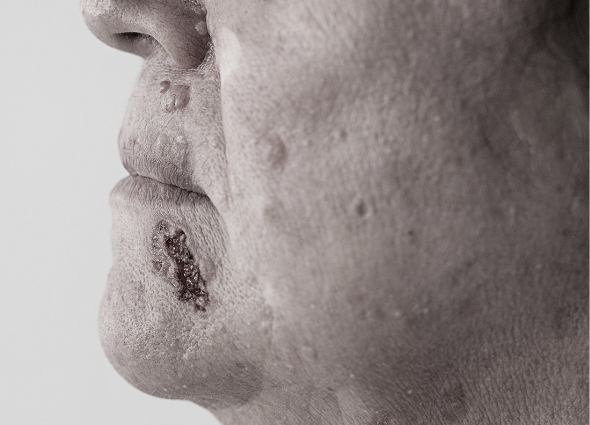
SGT-210
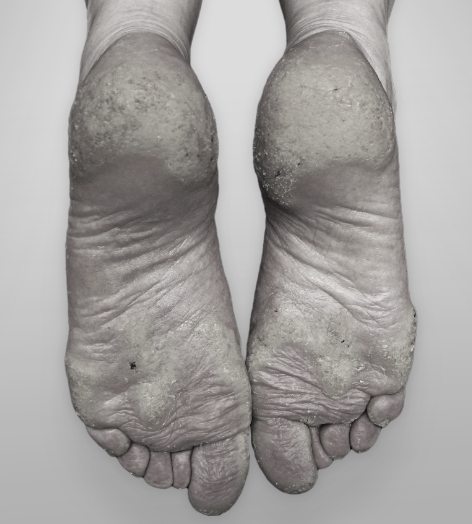
SGT-210 is our topical erlotinib drug candidate that is expertly formulated for the treatment of pachyonychia congenita (PC) and other hyperkeratosis-related indications.
Erlotinib is a tyrosine kinase receptor inhibitor that acts on the epidermal growth factor receptor, a protein present on cell surfaces that plays a key role in promoting cell growth and division.4 PC and other hyperkeratosis indications are characterized by thickened skin of the palms of the hands and soles of the feet, thickened nails, and other painful symptoms.
SGT-210 has the potential to be the first and only topical erlotinib designed to reduce hyperkeratosis with minimal systemic adverse events.


Harnessing topical formulation expertise
Drawing on our technical expertise, we overcame the topical formulation limitations of erlotinib and developed a topical product with a significantly higher concentration of erlotinib than what was previously reported to be inefficient.
Partnerships
Sol-Gel is seeking to expand its pipeline of dermatological drug products by acquiring proven drug candidates. We are also open to opportunities to license out our proprietary microencapsulation technology for improving the efficacy and/or tolerability of currently marketed dermatological drugs.
If you own a dermatology drug candidate in the clinical stage and you are looking to expedite development, or if you are interested in gaining further patent protection, we’re here to help.
For partnership inquiries, please send us a message on our Contact page.


References:
- Bakshi A, Chaudhary SC, Rana M, Elmets CA, Athar M. Basal cell carcinoma pathogenesis and therapy involving hedgehog signaling and beyond. Mol Carcinog. 2017;56(12):2543-2557. doi:10.1002/mc.22690
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366(23):2180-2188. doi:10.1056/NEJMoa1113538
- Nguyen NM, Cho J. Hedgehog pathway inhibitors as targeted cancer therapy and strategies to overcome drug resistance. Int J Mol Sci. 2022;23(3):1733. doi:10.3390/ijms23031733
- Siegel-Lakhai WS, Beijnen JH, Schellens JHM. Current knowledge and future directions of the selective epidermal growth factor receptor inhibitors erlotinib (Tarceva) and gefitinib (Iressa). Oncologist. 2005;10(8):579-589. doi:10.1634/theoncologist.10-8-579